What Does It Mean to Say That Animals Live in a Heterogeneous World That Varies Continuously
Introduction
Environmental heterogeneity is ubiquitous in natural systems and influences population dynamics and community structure1,2,3,4,5,6,7. Among ecological hypotheses relevant to environmental heterogeneity, the shape of the heterogeneity-diversity relationship (HDR) has been intensively explored in the past decades2,8,9,10,11,12,13. Based on the niche differentiation concept, a more heterogeneous environment could support more species through partitioned niche space1,2,3,14,15,16,17,18,19, which implies a positive HDR. Though positive HDRs have been widely documented in literature, several studies have recently questioned the generality of this pattern20,21,22,23,24. Other types of HDR, including negative, unimodal and non-significant, have been frequently reported as well24,25,26,27,28.
In plant communities, mean resource levels can affect species richness at fine spatial scales9. Many experimental studies have separated the effects of resource heterogeneity from average resource availabilities25, but the influence of spatial environmental heterogeneity across longer gradients of habitat fertility has been little examined. A previous study showed that intra-plot environmental heterogeneity should allow for more species to coexist in plots than predicted by mean levels of environmental variables29. Multiple factors, such as different measures of heterogeneity and spatial scale12,13,25,26, have been invoked to explain variation in the shape of HDRs. However, the effect of average resource availability on HDRs has been completely overlooked in previous studies of environmental gradients26.
Many studies have shown that environmental severity can exert a strong influence on species richness1,30,31. In temperate and boreal biomes, the relationship between plant species richness and biomass tends to be unimodal, whereas in the tropics it is often monotonically increasing32. Mechanisms determining these patterns are still debated33,34,35. To explain unimodal richness-biomass patterns, Grime invoked local plant interactions (competition) as reducing diversity in situations with low environmental severity, whereas richness declines in areas with high environmental severity due to lower numbers of species in regional pools that can tolerate stressful conditions30. Others have suggested that smaller regional species pools for the extremes of environmental gradients combined with larger pools for intermediate environmental conditions can cause unimodal diversity patterns36,37. Varying dispersal limitations for species associated with different parts of environmental gradients may also contribute to richness patterns38.
The relationships between environmental heterogeneity and species diversity across environmental gradients should depend on the mechanisms governing the overall relationship between mean environmental severity and species richness and the effect of heterogeneity on diversity at a given level of mean environmental severity.
More heterogeneous patches will contain a greater range of conditions at the same mean level of environmental severity. The increasing range of environmental conditions, however, does not necessarily result in higher species richness. In fact, the impact of the increasing range of environmental conditions on biodiversity will largely depend on the relationship between environmental severity and richness, as the number of species each heterogeneous patch can support corresponds to a specific location with the same level of environmental severity of this heterogeneous patch on the environmental severity-richness relationship obtained from homogeneous landscapes. This implies that the shape of the HDR could vary along environmental gradients. However, no studies have explored this hypothesis, theoretically or experimentally.
In the present paper, through an individual-based spatially explicit model based on previous studies39,40, we control the level of resource availability and isolate the effect of environmental heterogeneity from that of resource availability on community structure. This model simulates communities of sessile organisms and generates unimodal species richness-environmental severity relationships, based on the trade-off between stress-tolerance and competitive ability30. In the model, environmental severity is negatively associated with resource availability (represented by a variable S in the model)39, i.e. environmental severity is maximal at the lowest resource availability or productivity. The shape of the species richness-environmental severity relationship in this model is fairly robust to variation in species pool size40. We hypothesized that the shape of HDR would depend on the location of communities along environmental gradients: at either end of the environmental gradient, heterogeneity should promote greater richness, i.e. a positive HDR, as it will increase the prevalence of patches with intermediate environmental severity which draw from the largest species pool; at intermediate severity levels, heterogeneity could promote lower richness, i.e. a negative HDR, as patches will be present that draw from the smaller species pools associated with environmental extremes.
Results
On the homogenous landscapes without heterogeneity, as displayed by other studies using this model39,40, there existed a hump-shaped relationship between environmental severity and species richness, with the highest value occurring at intermediate environmental conditions (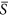 is about 0.4) (Fig. 1). However, the curve shapes within each half of this humped relationship were different. For the left half of this hump-shaped curve (
is about 0.4) (Fig. 1). However, the curve shapes within each half of this humped relationship were different. For the left half of this hump-shaped curve (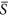 is from 0.0 to 0.4), the change of species richness (i.e. the slope of the curve at a given environmental severity) arose as an increasing function with environmental severity. For the right half of the curve (
is from 0.0 to 0.4), the change of species richness (i.e. the slope of the curve at a given environmental severity) arose as an increasing function with environmental severity. For the right half of the curve (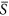 is from 0.4 to 1.0), however, the change of species richness was relatively constant with environmental severity.
is from 0.4 to 1.0), however, the change of species richness was relatively constant with environmental severity.
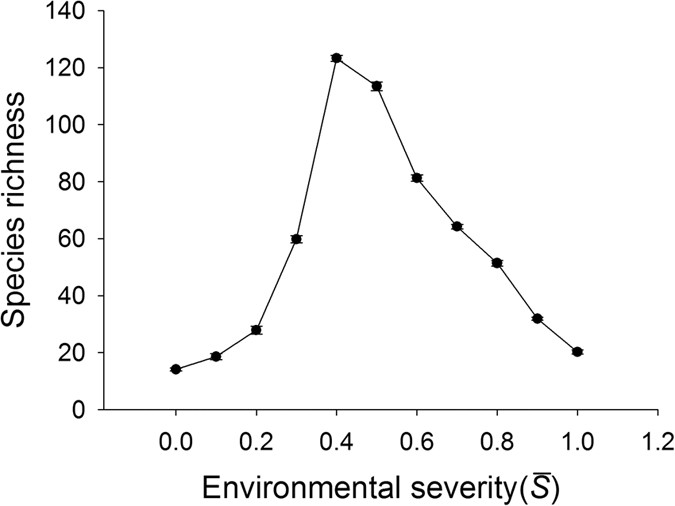
Change of species richness along the environmental severity gradient.
Landscapes were homogeneous with the size of 100 × 100 cells. Each data point represents the mean ± SE (N = 10). The parameter values used in models are r max = 1, r min = 0.2, r s = 0.1 and c = 1.
All comparisons relevant to the heterogeneous landscapes were under the constraint of the equal average level of environmental severity. In the case of the average environmental severity equal to 0.25 and 0.75 (Fig. 2a,b), there was a positive trend between species richness and environmental heterogeneity (represented by the standard deviation of S k across patches), though it was not substantial. In contrast, when the average environmental severity was equal to 0.40 and 0.50, species richness first increased then decreased with the standard deviation of S k across patches, leading to a unimodal relationship between richness and environmental heterogeneity (Fig. 2c,d).
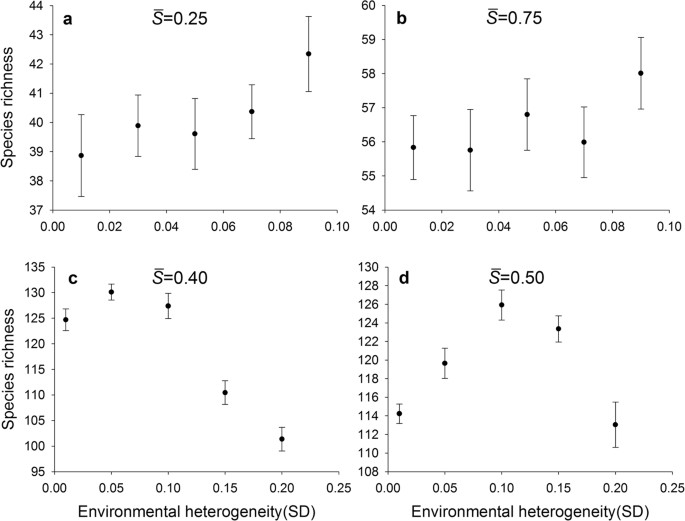
Comparison of species richness-environmental heterogeneity relationships.
Environmental heterogeneity was represented by the standard deviation of S k values across patches. The environmental severity (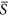 ) was 0.25 in (a), 0.75 in (b), 0.40 in (c) and 0.50 in (d). The whole landscape was divided into 400 patch types. Each data point represents the mean ± SE (N = 10).
) was 0.25 in (a), 0.75 in (b), 0.40 in (c) and 0.50 in (d). The whole landscape was divided into 400 patch types. Each data point represents the mean ± SE (N = 10).
Discussion
When mean environmental severity or resource availability was held constant, our results demonstrated that the shape of the HDR was influenced by the positions of communities along the resource availability gradient: a positive HDR when resource availability was near the ends of the environmental gradient and a unimodal HDR at the intermediate levels of environmental severity.
These divergent patterns could be largely explained by the relationship between species richness and environmental severity on homogeneous landscapes (Fig. 1). For example, in the case where the average environmental severity was 0.25 (Fig. 2a), richness increased with increasing heterogeneity (SD). Along that range of environmental severity under homogeneous conditions (Fig. 1), richness increased strongly with environmental severity between 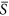 = 0.2 and 0.3, but less strongly between
= 0.2 and 0.3, but less strongly between 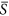 = 0.1 and 0.2. Thus under heterogeneous conditions at environmental severity
= 0.1 and 0.2. Thus under heterogeneous conditions at environmental severity 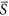 = 0.25 (Fig. 2a), the increase of species richness in patches with
= 0.25 (Fig. 2a), the increase of species richness in patches with 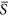 values approaching 0.50 was partly offset by the decrease of species richness in patches with
values approaching 0.50 was partly offset by the decrease of species richness in patches with 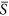 values approaching 0.0. Consequently, environmental heterogeneity led to an increase in species richness (Fig. 2a). In a similar way, for the case of
values approaching 0.0. Consequently, environmental heterogeneity led to an increase in species richness (Fig. 2a). In a similar way, for the case of 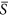 = 0.75, there also existed a positive trend in HDR (Fig. 2b). The results with the positive HDR are consistent with many previous studies12,25,26,41 and support our hypothesis that a positive HDR would occur at the ends of the environmental gradient.
= 0.75, there also existed a positive trend in HDR (Fig. 2b). The results with the positive HDR are consistent with many previous studies12,25,26,41 and support our hypothesis that a positive HDR would occur at the ends of the environmental gradient.
Our hypothesis that a negative HDR would emerge at the intermediate levels of the environmental gradient was partly supported by the simulation results. Actually, a unimodal rather than monotonic HDR occurred (Fig. 2c,d). Take 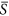 = 0.40 as an example. When the standard deviation was small, such as 0.01, the S k values for 400 patches were closely clustered around the mean 0.4, covering a very small range of potential environmental niches. The hump-shaped curve of species richness versus environmental severity (Fig. 1) implies that for a given area, a patch characterized by an intermediate level of environmental severity would support more species than the one characterized by other levels of severity. With the increase of the standard deviation, the landscape spanned a wider environmental range, resulting in higher species richness. However, with the further increase of the standard deviation, each patch had an increasing probability to be assigned an S k value near two ends of the gradient. Thus, the increasing proportion of patches on the landscape with S k values near two ends of the gradient inevitably led to a decrease in species richness (Fig. 1).
= 0.40 as an example. When the standard deviation was small, such as 0.01, the S k values for 400 patches were closely clustered around the mean 0.4, covering a very small range of potential environmental niches. The hump-shaped curve of species richness versus environmental severity (Fig. 1) implies that for a given area, a patch characterized by an intermediate level of environmental severity would support more species than the one characterized by other levels of severity. With the increase of the standard deviation, the landscape spanned a wider environmental range, resulting in higher species richness. However, with the further increase of the standard deviation, each patch had an increasing probability to be assigned an S k value near two ends of the gradient. Thus, the increasing proportion of patches on the landscape with S k values near two ends of the gradient inevitably led to a decrease in species richness (Fig. 1).
Negative HDRs have been a key topic in recent studies16,20,22,27,28. If high heterogeneity also results in smaller habitats characterized by similar conditions, then overall richness can decline due to stochastic extinctions42. This is now called "microfragmentation" and is invoked to explain negative HDRs20,22,26,27,28. Within a fixed total community size, species richness decreases due to the stochastic extinction at high levels of environmental heterogeneity because the area available per habitat decreases43. However, the mechanism of microfragmentation did not operate in our simulations, as the number of patches (400 patches) and the area of landscape (100 × 100 cells) were both fixed. This implies that the present study provides a novel explanation for the negative HDR, which needs further empirical tests. Additionally, species richness is usually closely associated with the spatial extent of the study44,45. One potential refinement of our model could explore the combined effects of landscape area, resource availability and heterogeneity on community dynamics and their interactions.
Our results demonstrate that the shape of the relationship between species richness and environmental heterogeneity strongly depends on the positions of communities located on gradients of environmental severity. The experimental tests for predictions explored here could be easily implemented in field. A recent experimental study27 provides a good example showing how to isolate the confounding effect of resource availability from resource heterogeneity on species richness27. Additionally, because growth rate (and likely, the rates of competitive exclusion) and individual size (individual plants are larger in lower environmental severity) usually change with the environmental gradient46, a refined version of our model should explicitly consider plant size and the potential relationship between the spatial scale and the size of individual plants25,26,47. In the present work, we tested the change of HDR in the context of the unimodal environmental severity-diversity pattern. Another prospective improvement of the model is to explore the potential influence of various environmental severity-diversity relationships on HDRs, to determine the generality of the conclusions obtained here.
Methods
Our model involves a long-recognized trade-off between competitive ability and stress tolerance of plant species30,37,39,48,49,50,51,52,53. In the model, species display a strategy-dependent distribution along the environmental gradient: Those with strong competitive ability would dominate communities in benign conditions (low environmental severity), while ones with strong stress-tolerant ability would dominate communities in harsh conditions (high environmental severity). This model supplies a unique position for us to test HDR, as it explicitly incorporates the linkage between the strategy of life history closely associated with species' niche requirements and environmental gradients.
In our model, environmental severity was patch-specific (the variable S k was designated for patch k), with the range from 0 (i.e. the most benign environment) to 1 (i.e. the most severe environment). A variable p j, i represents the competitive ability of individual j of species i. To obtain p j, i values, we firstly randomly drew a value for each species (p i ) from a uniform distribution [0, 1] with the interval of 0.005 (1 divided by the size of regional species pool). This set of values for all species represents inter-specific variation. Then, for each individual of a given species, we randomly drew a value from a normal distribution, with the mean equal to p i and the standard deviation of 0.003. Through this, we incorporated intra-specific variation into the model. The results from skewed distributions of competitive abilities were qualitatively similar with the ones presented here (see the Supplementary Figs S1–S4 online). The larger the p j, i is, the higher the probability that species i invades a neighboring cell occupied by another species. We also assumed that any species could invade the empty cells. To account for the tradeoff, we assumed that the reproduction rate (r) of competitive species declines more rapidly with increasing environmental severity than that of stress-tolerant species:

where r max and r min refer to the maximum and minimum reproductive rate, respectively, which were equal among species and c was a constant greater than 0, indicating the linear negative correlation between the competitive ability and the fecundity of species. Though it is possible to set species-specific r max and r min , we have no sufficient prior information from literature to determine the relationships between r max and r min and corresponding competitive ability of species. When an individual's reproductive rate r was negative but greater than a threshold r s , it could reproduce but still persisted in the community. If an individual's reproductive rate r was more negative than r s , the individual died39,54.
Simulation modeling was executed on a two-dimensional square landscape with the size of 100 × 100 cells. Each cell can be empty or occupied by only one individual plant. Different measures for environmental heterogeneity have been proposed in literature12,13, including diversity of land cover types16, elevation range22 and the standard deviation of a specific environmental variable55. In the present paper, without loss of generality, we quantified environmental heterogeneity through the standard deviation of S k across patches on the landscape42, thus heterogeneity and species diversity were evaluated for the entire landscape. Thus, the homogenous landscape means that the standard deviation of S k across patches was equal to 0. We explored the change of species richness along the environmental gradient on these homogeneous landscapes.
In terms of heterogeneity, we divided the landscape into 400 patches, with each patch having 25 cells (the results from the case with 2500 patches were qualitatively similar with the ones presented here). To test whether HDR changes along the environmental gradient, we considered four levels of resource availability without loss of generality, i.e. 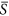 = 0.25, 0.40, 0.50 and 0.75. Via changing the standard deviation, we obtained the different levels of heterogeneity. Hence, for each patch, the variable of environmental severity, i.e. S k , was randomly drawn from a normal distribution with
= 0.25, 0.40, 0.50 and 0.75. Via changing the standard deviation, we obtained the different levels of heterogeneity. Hence, for each patch, the variable of environmental severity, i.e. S k , was randomly drawn from a normal distribution with 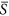 parameterized here and the standard deviation of interest. In other words, patches differed with respect to S k values, but cells within a given patch shared the identical value. For the cases of
parameterized here and the standard deviation of interest. In other words, patches differed with respect to S k values, but cells within a given patch shared the identical value. For the cases of 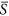 = 0.25 and 0.75, we set the standard deviation as 0.01, 0.03, 0.05, 0.07 and 0.09. For the case of
= 0.25 and 0.75, we set the standard deviation as 0.01, 0.03, 0.05, 0.07 and 0.09. For the case of 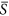 = 0.40 and S = 0.50, standard deviation was set as 0.01, 0.05, 0.10, 0.15 and 0.20. Such settings of standard deviation guarantee that most values randomly drawn from normal distributions fall into the range of the environmental gradient [0, 1].
= 0.40 and S = 0.50, standard deviation was set as 0.01, 0.05, 0.10, 0.15 and 0.20. Such settings of standard deviation guarantee that most values randomly drawn from normal distributions fall into the range of the environmental gradient [0, 1].
The initial landscape was saturated and occupied by the same number of species as in regional species pool (R = 200 here; the size of regional species pool will not qualitatively change the results), with the number of individuals for each species following a negative exponential distribution. The model sequentially ran through the following modules: mortality, immigration and reproduction and dispersal.
Mortality
All individuals in the community suffered from a given degree of environmental stochasticity and disturbance. To remove the potential confounding effect of mortality from environmental heterogeneity, we assumed that individuals from all species experience the same probability of death due to stochastic factors.
Immigration
In each iteration, we randomly selected a fixed number (I = 10; results are qualitatively similar with I = 20) of individuals from the regional species pool R. These selected individuals were randomly assigned to cells across the whole landscape. When the cell an individual reached was occupied by another individual, the relative competitive ability between species to which the two individuals belonged determined whether the invading individual could exclude the resident individual and occupy the target cell. For instance, for individual m of resident species k and individual j of invading species i, if p j,i −p m,k was greater than a random value drawn from a uniform distribution from 0 to 1, the individual of species i replaced the individual of species j.
Reproduction and dispersal
Equation 1 determined the reproductive rate r j,i for each individual. As r j,i is not always an integer, we calculated the number of offspring with the following rule: If the fractional part of r j,i is greater than a random value drawn from a uniform distribution ([0, 1]), the number of offspring is equal to the integer part of r j,i plus 1. The competitive ability of offspring was identical to the parent. These newly born propagules were randomly dispersed to the neighboring eight cells of the parent. We assumed that the dispersal can occur within each patch, or among patches. Similar to the above rule of immigration, if the cell the propagule reached was occupied by an individual of another species, the competitive ability between them determined the final outcome. In addition, the propagules of all species could successfully invade the empty cells.
Simulations were run 10 000 steps in order to allow the community to approach a dynamical equilibrium state. Community composition, including species identity and abundance, was recorded in 200 step intervals for each setting of parameters after the 10 000 startup steps. We conducted 10 replicates for each setting of parameters. Simulations were implemented in NetLogo (v5.0.4) software56 and a "wrap-around" approach was used to avoid edge effects57.
Additional Information
How to cite this article: Yang, Z. et al. The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci. Rep. 5, 15723; doi: 10.1038/srep15723 (2015).
References
-
Tilman, D. Resource Competition and Community Structure (Princeton University Press, 1982).
-
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000).
-
Amarasekare, P. Competitive coexistence in spatially structured environments: A synthesis. Ecol. Lett. 6, 1109–1122 (2003).
-
Maestre, F. T. & Reynolds, J. F. Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Glob. Change Biol. 12, 2431–2441 (2006).
-
Oliver, T., Roy, D. B., Hill, J. K., Brereton, T. & Thomas, C. D. Heterogeneous landscapes promote population stability. Ecol. Lett. 13, 473–484 (2010).
-
Eilts, J. A., Mittelbach, G. G., Reynolds, H. L. & Gross, K. L. Resource heterogeneity, soil fertility and species diversity: Effects of clonal species on plant communities. Am. Nat. 177, 574–588 (2011).
-
Chisholm, R. A. et al. Temporal variability of forest communities: Empirical estimates of population change in 4000 tree species. Ecol. Lett. 17, 855–865 (2014).
-
Reynolds, H. L., Hungate, B. A., Chapin, F. S. & D'antonio, C. M. Soil heterogeneity and plant competition in an annual grassland. Ecology 78, 2076–2090 (1997).
-
Stevens, M. H. H. & Carson, W. P. Resource quantity, not resource heterogeneity, maintains plant diversity. Ecol. Lett. 5, 420–426 (2002).
-
Questad, E. J. & Foster, B. L. Coexistence through spatio-temporal heterogeneity and species sorting in grassland plant communities. Ecol. Lett. 11, 717–726 (2008).
-
Brown, C. et al. Multispecies coexistence of trees in tropical forests: Spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proc. R. Soc. B-Biol. Sci. 280, 326–348 (2013).
-
Stein, A., Gerstner, K. & Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880 (2014).
-
Stein, A. & Kreft, H. Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev. 90, 815–836 (2014).
-
Chesson, P. L. & Warner, R. R. Environmental variability promotes coexistence in lottery competitive systems. American Naturalist 117, 923–943 (1981).
-
Kohn, D. D. & Walsh, D. M. Plant species richness-the effect of island size and habitat diversity. J. Ecol. 82, 367–377 (1994).
-
Hortal, J., Triantis, K. A., Meiri, S., Thebault, E. M. C. & Sfenthourakis, S. Island species richness increases with habitat diversity. Am. Nat. 174, E205–E217 (2009).
-
Smith, T. W. & Lundholm, J. T. Environmental geometry and heterogeneity-diversity relationships in spatially explicit simulated communities. J. Veg. Sci. 23, 732–744 (2012).
-
Price, J. N., Gazol, A., Tamme, R., Hiiesalu, I. & Pärtel, M. The functional assembly of experimental grasslands in relation to fertility and resource heterogeneity. Funct. Ecol. 28, 509–519 (2014).
-
Weisberg, P. J. et al. Guild-specific responses of avian species richness to lidar-derived habitat heterogeneity. Acta Oecol.-Int. J. Ecol. 59, 72–83 (2014).
-
Kadmon, R. A. & Allouche, O. Integrating the effects of area, isolation and habitat heterogeneity on species diversity: A unification of island biogeography and niche theory. Am. Nat. 170, 443–454 (2007).
-
Allouche, O. & Kadmon, R. A general framework for neutral models of community dynamics. Ecol. Lett. 12, 1287–1297 (2009).
-
Allouche, O., Kalyuzhny, M., Moreno-Rueda, G., Pizarro, M. & Kadmon, R. Area-heterogeneity tradeoff and the diversity of ecological communities. Proc. Natl. Acad. Sci. USA 109, 17495–17500 (2012).
-
Schoolmaster, D. R. Jr. Resource competition and coexistence in heterogeneous metacommunities: Many-species coexistence is unlikely to be facilitated by spatial variation in resources. PeerJ 1, e136, 10.7717/peerj.136 (2013).
-
Bar-Massada, A. & Wood, E. M. The richness-heterogeneity relationship differs between heterogeneity measures within and among habitats. Ecography 37, 528–535 (2014).
-
Lundholm, J. T. Plant species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. J. Veg. Sci. 20, 377–391 (2009).
-
Tamme, R., Hiiesalu, I., Laanisto, L., Szava-Kovats, R. & Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 21, 796–801 (2010).
-
Gazol, A. et al. A negative heterogeneity-diversity relationship found in experimental grassland communities. Oecologia 173, 545–555 (2013).
-
Laanisto, L. et al. Microfragmentation concept explains non-positive environmental heterogeneity-diversity relationships. Oecologia 171, 217–226 (2013).
-
Palmer, M. W. & Dixon, P. M. Small-scale environmental heterogeneity and the analysis of species distributions along gradients. J. Veg. Sci. 1, 57–65 (1990).
-
Grime, J. P. Competitive exclusion in herbaceous vegetation. Nature 242, 344–347 (1973).
-
Waide, R. B. et al. The relationship between productivity and species richness. Annu. Rev. Ecol. Syst. 30, 257–300 (1999).
-
Pärtel, M., Laanisto, L. & Zobel, M. Contrasting plant productivity-diversity relationships across latitude: The role of evolutionary history. Ecology 88, 1091–1097 (2007).
-
Pan, X., Liu, F. & Zhang, M. Comment on "Productivity is a poor predictor of plant species richness". Science 335, 1441 (2012).
-
Adler, P. B. et al. Productivity is a poor predictor of plant species richness. Science 333, 1750–1753 (2011).
-
Grace, J. B. et al. Response to comments on "Productivity is a poor predictor of plant species richness". Science 335, 1441–1441 (2012).
-
Taylor, D. R., Aarssen, L. W. & Loehle, C. On the relationship between r/k selection and environmental carrying capacity: A new habitat templet for plant life history strategies. Oikos 58, 239–250 (1990).
-
Schamp, B., Aarssen, L. & Lee, H. Local plant species richness increases with regional habitat commonness across a gradient of forest productivity. Folia Geobot. 38, 273–280 (2003).
-
Zobel, M. & Pärtel, M. What determines the relationship between plant diversity and habitat productivity? Glob. Ecol. Biogeogr. 17, 679–684 (2008).
-
Xiao, S., Michalet, R., Wang, G. & Chen, S.-Y. The interplay between species' positive and negative interactions shapes the community biomass-species richness relationship. Oikos 118, 1343–1348 (2009).
-
Xiao, S., Zobel, M., Szava-Kovats, R. & Pärtel, M. The effects of species pool, dispersal and competition on the diversity-productivity relationship. Glob. Ecol. Biogeogr. 19, 343–351 (2010).
-
Lundholm, J. T. & Larson, D. W. Relationships between spatial environmental heterogeneity and plant species diversity on a limestone pavement. Ecography 26, 715–722 (2003).
-
Palmer, M. W. The coexistence of species in fractal landscapes. Am. Nat. 139, 375–397 (1992).
-
Hortal, J. et al. Species richness can decrease with altitude but not with habitat diversity. Proc. Natl. Acad. Sci. USA 110, E2149–E2150 (2013).
-
Harte, J. & Green, J. Self-similarity in the distribution and abundance of species. Science 284, 334–336 (1999).
-
Preston, F. W. The canonical distribution of commonness and rarity: Part II. Ecology 43, 185–215 (1962).
-
Harper, J. & White, J. The demography of plants. Annu. Rev. Ecol. Syst. 5, 419–463 (1974).
-
Weiner, J. The effects of density, spatial pattern and competitive symmetry on size variation in simulated plant populations. Am. Nat. 158, 438–450 (2001).
-
Grime, J. P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194 (1977).
-
Molino, J.-F. & Sabatier, D. Tree diversity in tropical rain forests: A validation of the intermediate disturbance hypothesis. Science 294, 1702–1704 (2001).
-
Bakker, J. P. Competitors, ruderals and stress-tolerators. New Phytol. 156, 6–8 (2002).
-
Liancourt, P., Callaway, R. M. & Michalet, R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 86, 1611–1618 (2005).
-
Michalet, R. et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773 (2006).
-
Peterson, M. L., Rice, K. J. & Sexton, J. P. Niche partitioning between close relatives suggests trade-offs between adaptation to local environments and competition. Ecol. Evol. 3, 512–522 (2013).
-
Wang, Y. et al. The effect of positive interactions on temporal turnover of community composition along an environmental gradient. PLoS ONE 8, e78698, 10.1371/journal.pone.0078698 (2013).
-
Balvanera, P. & Aguirre, E. Tree diversity, environmental heterogeneity and productivity in a Mexican tropical dry forest. Biotropica 38, 479–491 (2006).
-
Wilensky, U. & Evanston, I. NetLogo: Center for connected learning and computer-based modeling. Northwestern University, Evanston, IL (1999) Date of access: 01/04/2014. Available at: http://ccl.northwestern.edu/netlogo/.
-
Grimm, V. & Railsback, S. F. Individual-based Modeling and Ecology (Princeton University Press, 2005).
Acknowledgements
We are very grateful to Anke Stein for the constructive comments on an earlier version of this manuscript. The work was financially supported by the National Natural Science Foundation of China (41201050, 31570426 and 31000199) and the Fundamental Research Funds for the Central Universities (lzujbky-2013-k15). CC also benefited from the Program for New Century Excellent Talents in University (NCET-12-0248). JTL was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Contributions
Z.Y., G.W., Y.W. and C.C. conceived and designed the simulation experiments. Z.Y., X.L., M.Z. and D.A. performed the simulation experiments. J.T.L. provided valuable suggestions on the simulation scenarios. Z.Y., Y.W., C.C. and J.T.L. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and Permissions
About this article
Cite this article
Yang, Z., Liu, X., Zhou, M. et al. The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci Rep 5, 15723 (2015). https://doi.org/10.1038/srep15723
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/srep15723
delacruzfitain1956.blogspot.com
Source: https://www.nature.com/articles/srep15723
0 Response to "What Does It Mean to Say That Animals Live in a Heterogeneous World That Varies Continuously"
Post a Comment